Potent and selective A3 adenosine receptor antagonists bearing aminoesters as heterobifunctional moieties†
Abstract
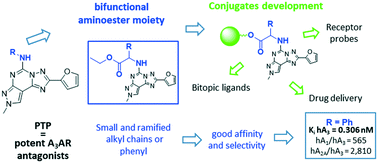
A3 adenosine receptors were found to have a role in different pathological states, such as glaucoma, renal fibrosis, neuropathic pain and cancer. Consequently, it is important to utilize any molecular tool which could help to study these conditions. In the present study we continue our search for potent A3 adenosine receptor ligands which could be successively conjugated to other molecules with the aim of obtaining more potent (e.g. allosteric ligand conjugation) or detectable ligands (e.g. fluorescent molecule or biotin conjugation). Specifically, different aminoester moieties were introduced at the 5 position of the pyrazolo[4,3-e]-1,2,4-triazolo[1,5-c]pyrimidine core. The ester functionalization represents the candidate for the subsequent conjugation. All the reported compounds are potent hA3 adenosine receptor antagonists and some of them exhibited high selectivity against the other adenosine receptors. The main structural terms of ligand recognition and selectivity were disclosed by molecular modelling studies. Molecular docking results led to the characterization of an alternative binding mode for antagonists at the orthosteric binding site of the hA3 adenosine receptor, evaluated and assessed by classical molecular dynamics simulations.
- This article is part of the themed collection: Emerging Investigators


 Please wait while we load your content...
Please wait while we load your content...