N-Butylpyrrolidone (NBP) as a non-toxic substitute for NMP in iron-catalyzed C(sp2)–C(sp3) cross-coupling of aryl chlorides†
Abstract
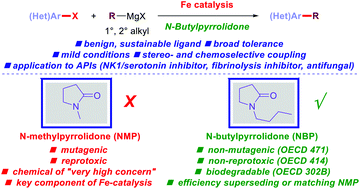
Although iron catalyzed cross-coupling reactions show extraordinary promise in reducing the environmental impact of more toxic and scarce transition metals, one of the main challenges is the use of reprotoxic NMP (NMP = N-methylpyrrolidone) as the key ligand to iron in the most successful protocols in this reactivity platform. Herein, we report that non-toxic and sustainable N-butylpyrrolidone (NBP) serves as a highly effective substitute for NMP in iron-catalyzed C(sp2)–C(sp3) cross-coupling of aryl chlorides with alkyl Grignard reagents. This challenging alkylation proceeds with organometallics bearing β-hydrogens with efficiency superseding or matching that of NMP with ample scope and broad functional group tolerance. Appealing applications are demonstrated in the cross-coupling in the presence of sensitive functional groups and the synthesis of several pharmaceutical intermediates, including a dual NK1/serotonin inhibitor, a fibrinolysis inhibitor and an antifungal agent. Considering that the iron/NMP system has emerged as one of the most powerful iron cross-coupling technologies available in both academic and industrial research, we anticipate that this method will be of broad interest.
- This article is part of the themed collection: 2021 Green Chemistry Hot Articles


 Please wait while we load your content...
Please wait while we load your content...
