Formic acid disproportionation into formaldehyde triggered by vanadium complexes with iridium catalysis under mild conditions in N-methylation†
Abstract
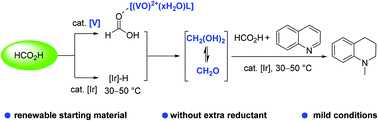
Formaldehyde (CH2O) has been used as a key platform reagent in the chemical industry for many decades. Currently, the industrial production of CH2O mainly depends on fossil resources, involving a highly energetic three-step process (200–1100 °C). Herein, we describe renewable formic acid (HCO2H) disproportionation into CH2O triggered by vanadium complexes with iridium catalysis under mild conditions at 30–50 °C in N-methylation. The gram-scale application of in situ generated CH2O by HCO2H disproportionation is demonstrated.
- This article is part of the themed collection: Sustainable synthesis and catalysis – Chemical Science symposium collection


 Please wait while we load your content...
Please wait while we load your content...