Nanopore-based measurement of the interaction of P450cam monooxygenase and putidaredoxin at the single-molecule level†
Abstract
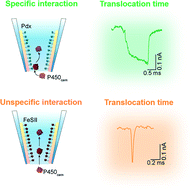
Protein–protein interactions occur in a wide range of biological processes and are of great significance to life function. Characterization of transient protein–protein interactions remains a significant barrier to our understanding of cellular processes. Nanopores provide unique nanoscale environments that accommodate single molecules from the surrounding bulk solution. This method permits label-free sensing at the single-molecule level with extremely high sensitivity. Herein, the interaction between a single P450cam monooxygenase and its redox partner putidaredoxin (Pdx) was monitored via transient ionic current by using functionalized glass nanopores. Results show that the volume of P450cam determines the blockage current while the interactions between the P450cam and Pdx give a long blockage duration. Our glass nanopore sensor with adjustable diameter could be applied for real-time sensing of protein–protein interactions between individual proteins with a wide range of molecular weight.
- This article is part of the themed collection: Next Generation Nanoelectrochemistry


 Please wait while we load your content...
Please wait while we load your content...