Electrochemical carbon dioxide reduction in ionic liquids at high pressure†
Abstract
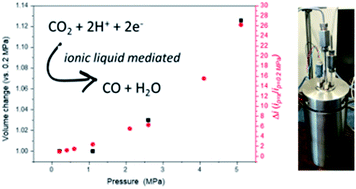
Imidazolium ionic liquids are potentially useful solvents for both carbon dioxide reduction conversion and capture. In particular electrocatalytic CO2 reduction has been shown to occur at low overpotentials using a 1-ethyl-3-methylimidazolium trifluoromethanesulfonate ([EMIM][OTf]) and water mixed solvent. A limitation of such solvent systems is their viscosity, making it hard to maintain reasonable catalytic current densities without energy intensive stirring/agitation of the electrolyte. Here we explore the electrochemical reduction of CO2 at high pressures (0.1 to 5.1 MPa) and demonstrate a correlation between the volume of expansion of the ionic liquid and the achieved catalytic current density. The improved electrocatalytic behaviour is proposed to be due to both the increased bulk CO2 concentration and the improved mass transport properties of the gas-expanded ionic liquid. These initial studies at pressure represent a step towards realising an integrated CO2 capture and utilisation system based around a common ionic liquid.
- This article is part of the themed collection: Carbon dioxide utilisation


 Please wait while we load your content...
Please wait while we load your content...