Promoting the uptake of chloride ions by ZnCo–Cl layered double hydroxide electrodes for enhanced capacitive deionization†
Abstract
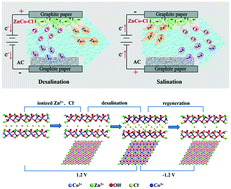
Hybrid capacitive deionization (HCDI) has gained a significant amount of attention in the field of desalination research owing to the remarkable salt removal capacity. In this work, a ZnCo–Cl layered double hydroxide (LDH) has been fabricated for use in high-performance HCDI anode by promoting the uptake of chloride ions. The electrochemical measurement demonstrates the typical conversion between the Co2+/Co3+ couple, accompanied by the intercalation/de-intercalation of Cl−. Moreover, the charge transfer resistance of the ZnCo–Cl LDH is only 0.15 Ω, which is conducive to improving the diffusion of salty ions and thereby the charge efficiency. When used as an anode, the salt removal capacity of the ZnCo–Cl LDH anode is 56.1 mg g−1 in 1000 μS cm−1 NaCl at 1.2 V. After 20 cycles, the capacity retention rate is maintained at 86%, suggesting superior regeneration. Beyond that, the post X-ray diffraction and X-ray photoelectron spectroscopy highlight the reversible intercalation/de-intercalation of Cl− in the desalination/salination process. As such, a possible HCDI mechanism enabled by the ZnCo–Cl LDH anode is proposed.
- This article is part of the themed collections: Nanomaterial applications in water and Environmental Science: Nano Recent HOT Articles


 Please wait while we load your content...
Please wait while we load your content...