High degradation efficiency of sulfamethazine with the dual-reaction-center Fe–Mn–SiO2 Fenton-like nanocatalyst in a wide pH range†
Abstract
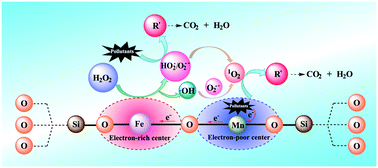
The development of a heterogeneous Fenton-like catalyst, possessing a high degradation efficiency in a wide pH range, is crucial for wastewater treatment. An Fe–Mn–SiO2 catalyst was designed and prepared by a hydrothermal method. Density functional theory (DFT) calculations demonstrated that the dual reaction centers were produced around the Fe and Mn atoms, due to valence-electron density distribution. The Fe–Mn–SiO2 catalyst with dual reaction centers was applied for sulfamethazine (SMT) degradation in the Fenton-like process at a pH value of 4.0–8.6. Almost 100% SMT degradation efficiency was achieved under neutral pH at room temperature within 180 min. Moreover, the Fe–Mn–SiO2/H2O2 system showed a high degradation efficiency (60–100%) for removing a wide range of aromatic pollutants, including bisphenol A, phenol, 4-chlorophenol, phenytoin, and ibuprofen. Besides, this process showed strong resistance to interference of inorganic anions (Cl−, SO42−, NO3− and HCO3−). Electron paramagnetic resonance and radical scavenger experiments confirmed that the generated 1O2 and O2˙−/HO2˙ played an important role in the degradation of organic pollutants. In the Fe–Mn–SiO2/H2O2 system, the electron-rich center around Fe was responsible for the reduction of H2O2 to 1O2, O2˙−/HO2˙ and ˙OH radicals, and then the electron-poor center around Mn adsorbed pollutants and transferred electrons to the electron-rich center around Fe via the Fe–O–Mn bridge.
- This article is part of the themed collection: Environmental Remediation


 Please wait while we load your content...
Please wait while we load your content...