Reciprocal redox interactions of lithium cobalt oxide nanoparticles with nicotinamide adenine dinucleotide (NADH) and glutathione (GSH): toward a mechanistic understanding of nanoparticle-biological interactions†
Abstract
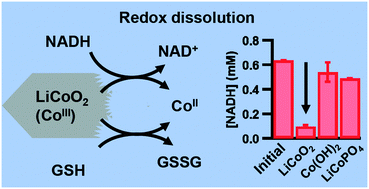
Among high-valence metal oxides, LiCoO2 and related materials are of environmental importance because of the rapidly increasing use of these materials as cathodes in lithium ion batteries. Understanding the impact of these materials on aqueous environments relies on understanding their redox chemistry because Co release is dependent on oxidation state. Despite the critical role that redox chemistry plays in cellular homeostasis, the influence of specific biologically relevant electron transporters such as nicotinamide adenine dinucleotide (NADH) and glutathione (GSH) on the transformation of engineered nanoparticles has not been widely considered previously. Here we report an investigation of the interaction of LiCoO2 nanoparticles with NADH and GSH. Measurements of Co release using inductively coupled plasma-mass spectrometry (ICP-MS) show that exposing LiCoO2 nanoparticles to either NADH or GSH increases solubilization of cobalt, while corresponding spectroscopic measurements show that NADH is concurrently oxidized to NAD+. To demonstrate that these effects are a consequence of the high-valence Co(III) in LiCoO2 nanoparticles, we performed control experiments using Co(II)-containing Co(OH)2 and LiCoPO4, and dissolved Co2+/Li+ ions. Additional experiments using molecules of similar structure to NADH and GSH, but that are not reducing agents, confirm that these transformations are driven by redox reactions and not by chelation effects. Our data show that interaction of LiCoO2 with NADH and GSH induces the release of Co2+ ions and alters the redox state of these biologically important transporters. Observation of NADH binding to LiCoO2 using X-ray photoelectron spectroscopy (XPS) suggests a surface catalyzed reaction. The reciprocal reduction of LiCoO2 to enable release of Co2+ and corresponding oxidation of NADH and GSH as model redox-active biomolecules has implications for understanding the biological impacts of high-valence metal oxide nanomaterials.
- This article is part of the themed collections: Environmental Science: Nano Recent HOT Articles, Best Papers of 2021 from RSC’s Environmental Science journals and Best Papers 2021 - Environmental Science: Nano


 Please wait while we load your content...
Please wait while we load your content...
