Acid and base strength variations: rationalization for cyclic amine bases and acidic aqua cations†
Abstract
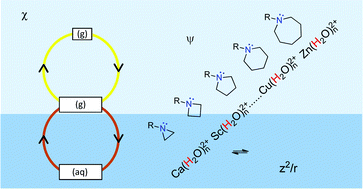
This perspective highlights and evaluates recent key developments in the thermodynamic approach used to analyze trends in acid and base strength variation. According to this approach, acid and base strength ranking can be interpreted by using thermodynamic or thermochemical cycles. Each cycle generally consists of three independent but well-defined steps. The modus operandi described here entails the identification of the dominant step and the rationalization of its free energy/enthalpy/energy change along a selected series in terms of known structural chemical concepts. Developments in this approach are described by focusing on two related series of bases and two series of acids. In the case of the former the protonation of a series of N-heterocyclic amine bases together with their methyl-substituted analogs receives particular attention while in the case of acids, the acidic properties of aqua dications of elements in period 4 and group 2 are probed. It is illustrated how significant progress in computational chemistry and mass spectrometric techniques can be employed to compare ‘inherent’ basicity or acidity in the selected families of compounds by using simple gas-phase energy cycles. Unique, dual functions for both electronegativity (element and orbital) and charge density (for aqua cations) indicators are identified and used to evaluate these cycles. Solvent effects (in aqueous solution) are accommodated by including dehydration and hydration changes in appropriately-extended, three-step free energy cycles. It is further suggested that the dominant step in the extended thermodynamic cycle for monomeric aqua cations is the transfer of M(H2O)n2+ complex hydrates from the gas-phase to bulk water. Charge density of the aqua cations again features prominently in proposed rationalizations. Finally, this article also sheds light on salient relationships that exist between empirically and quantum-chemically estimated enthalpy and entropy changes for the aforementioned transfer process.
- This article is part of the themed collection: 2021 Frontier and Perspective articles


 Please wait while we load your content...
Please wait while we load your content...