Proton controlled synthesis of two dicopper(ii) complexes and their magnetic and biomimetic catalytic studies together with probing the binding mode of the substrate to the metal center†
Abstract
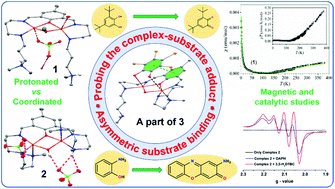
This paper describes the synthesis, and structural and spectroscopic characterizations of two doubly bridged dicopper(II) complexes, [Cu2(μ-H2L)(μ-OMe)](ClO4)4·2H2O (1) and [Cu2(μ-L)(μ-OH)](ClO4)2 (2), with a binucleating ligand (HL) derived from the Schiff base condensation of DFMP and N,N-dimethyldipropylenetriamine, and their biomimetic catalytic activities were related to CAO and phenoxazinone synthase using 3,5-di-tert-butylcatechol and o-aminophenol (OAPH), respectively, as model substrates. Structural studies reveal that the major differences in these structures appear to be from the distinct roles of the tertiary amine groups of the ligands, which are protonated in 1, whereas it coordinates the metal centers in 2. Magnetic studies disclose that two copper(II) centers are strongly antiferromagnetically coupled with slightly different J values, which is further interpreted and discussed. They exhibited very different biomimetic catalytic activities; whereas 2 is an efficient catalyst, complex 1 showed somewhat lower substrate oxidation. The higher reactivity in 2 is rationalized by the strong involvement of the tertiary amine group of the Schiff base ligand, where the substrate oxidation is favored because of the transfer of protons from the substrate to the tertiary amine group, showing the importance of the functional groups in proximity to the bimetallic active site. Emphasis was also given to probing the binding mode of the substrate using an electronically deficient tetrabromomocatechol (Br4CatH2) and the isolated compound [Cu6(μ-HL)2(μ-OH)2(Br4Cat)4](NO3)2·4H2O (3) which suggests that monodentate asymmetric binding of 3,5-di-tert-butylcatechol and OAPH occurs during the course of the catalytic reaction.


 Please wait while we load your content...
Please wait while we load your content...