Synthesis, structure and insertion reactivity of Lewis acidic 9-aluminafluorenes†
Abstract
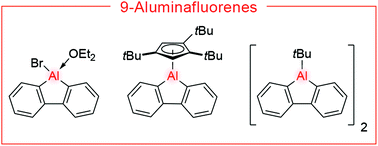
9-Aluminafluorenes have only been sparingly investigated and their properties still remain largely unexplored. Herein, we report the synthesis of five aluminafluorene derivatives with a diverse array of aluminium substituents and probe their Lewis acid properties and reactivity. We show that 9-bromo-9-aluminafluorene readily forms Lewis acid–base adducts with N-heterocyclic carbenes (NHCs), cyclic (alkyl)(amino)carbenes (CAACs) and pyridines and that it undergoes a selective ring expansion reaction with the iminoborane tBuN![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) BMes to give a seven-membered ring, which can be viewed as a boron–nitrogen analogue of alumepins.
BMes to give a seven-membered ring, which can be viewed as a boron–nitrogen analogue of alumepins.


 Please wait while we load your content...
Please wait while we load your content...