Hydroboration of carbonyls and imines by an iminophosphonamido tin(ii) precatalyst†
Abstract
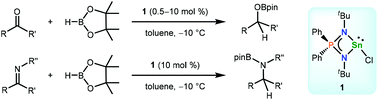
A novel three-coordinated tin(II) chloride [Ph2P(NtBu)2]SnCl (1) supported by an N,N′-di-tert-butyliminophosphonamide having two phenyl groups on the phosphorus atom was synthesized by the reaction of the starting lithium iminophosphonamide [Ph2P(NtBu)2]Li with SnCl2·(dioxane) in toluene. The molecular structure of 1 was established by X-ray diffraction analysis. Tin(II) chloride 1 can act as an efficient precatalyst for the hydroboration of a wide variety of aldehydes, ketones, and imines at −10 °C. DFT calculations propose that hydroboration involves hydride transfer from the corresponding tin(II) hydride intermediate [Ph2P(NtBu)2]SnH (10) to the carbonyl substrates via four-membered transition states (TS-12), affording three-coordinated tin(II) alkoxide intermediates [Ph2P(NtBu)2]SnOR (13), followed by the stepwise reaction of 13 with HBpin (pin = pinacolate) to release the boronate esters and regenerate the tin(II) hydride 10. The stoichiometric reaction of the in site-generated 10 with benzophenone 2a at −10 °C led to the formation of 13. Moreover, 13 also stoichiometrically reacted with HBpin at −10 °C, forming the corresponding boronate ester 3a and 10 based on the 1H NMR spectrum of the reaction mixture.
- This article is part of the themed collection: Tin: Modern chemistry of an element from antiquity


 Please wait while we load your content...
Please wait while we load your content...