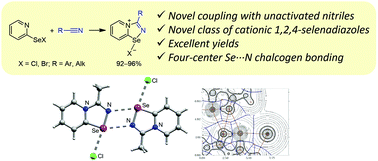
Novel cationic 1,2,4-selenadiazoles: synthesis via addition of 2-pyridylselenyl halides to unactivated nitriles, structures and four-center Se⋯N contacts†
Abstract
2-Pyridylselenyl halides undergo facile coupling with a triple CN bond of unactivated nitriles. Unprecedented heterocyclization allowed the preparation of a novel class of cationic 1,2,4-selenadiazoles in remarkably high yields. Cationic 1,2,4-selenadiazoles form supramolecular dimers in the crystal via Se⋯N chalcogen bonding, which was studied theoretically.


 Please wait while we load your content...
Please wait while we load your content...