Modulation of N^N′-bidentate chelating pyridyl–pyridylidene amide ligands offers mechanistic insights into Pd-catalysed ethylene/methyl acrylate copolymerisation†
Abstract
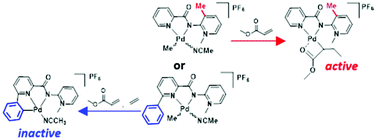
The efficient copolymerisation of functionalised olefins with alkenes continues to offer considerable challenges to catalyst design. Based on recent work using palladium complexes containing a dissymmetric N^N′-bidentate pyridyl-PYA ligand (PYA = pyridylidene amide), which showed a high propensity to insert methyl acrylate, we have here modified this catalyst structure by inserting shielding groups either into the pyridyl fragment, or the PYA unit, or both to avoid fast β-hydrogen elimination. While a phenyl substituent at the pyridyl side impedes catalytic activity completely and leads to an off-cycle cyclometallation, the introduction of an ortho-methyl group on the PYA side of the N^N′-ligand was more prolific and doubled the catalytic productivity. Mechanistic investigations with this ligand system indicated the stabilisation of a 4-membered metallacycle intermediate at room temperature, which has previously been postulated and detected only at 173 K, but never observed at ambient temperature so far. This intermediate was characterised by solution NMR spectroscopy and rationalises, in part, the formation of α,β-unsaturated esters under catalytic conditions, thus providing useful principles for optimised catalyst design.
- This article is part of the themed collection: Dalton turns 50 – celebrating our board members past and present


 Please wait while we load your content...
Please wait while we load your content...