Adjusting the CO2 hydrogenation pathway via the synergic effects of iron carbides and iron oxides†
Abstract
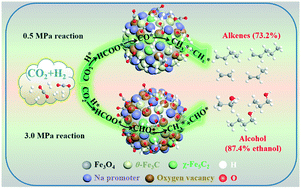
Owing to the chemical inertness and thermodynamic stability of CO2, efficiently regulating product selectivity during CO2 hydrogenation is still a big challenge. Without impairing the CO2 reactivity and stability, outstanding selectivity values, where 73.2% of the total hydrocarbon products were alkenes (CO free) and 97.9% of the total alcohol products were C2+ alcohols (87.4% ethanol), were achieved using a Na-promoted Fe3O4 microsphere catalyst. Significantly, compared to the literature results relating to CO2 hydrogenation, the highest yields of alkenes (22.03%) and ethanol (11.23%) were achieved in this work. Multiple characterization results demonstrate that the Na promoter contributes to tuning the composition of the iron oxides and iron carbides. In situ CO2-DRIFTS and NAP-XPS results revealed that the key step in ethanol formation is considered to be the bonding of adsorbed CHO* and *CHx. Synergistically utilizing the distinct catalytic roles of iron carbides and iron oxides can guide the design of effective catalysts and provide fundamental understanding relating to CO2 hydrogenation.


 Please wait while we load your content...
Please wait while we load your content...