CO2 reduction and ethane dehydrogenation on transition metal catalysts: mechanistic insights, reactivity trends and rational design of bimetallic alloys†
Abstract
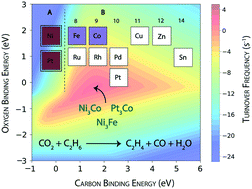
Reactivity trends of transition metal catalysts are studied for the ethane dehydrogenation reaction using CO2 as a mild oxidant. An ab initio microkinetic model (MKM) is constructed to gain insights about the dominant route for CO2 reduction and simultaneous ethylene formation over the terrace (111) and step (211) surfaces of the catalysts. At the terrace sites, Rh and Pt are observed to show high ethane consumption with maximum turnovers to produce ethylene. For CO2 consumption, Rh, Ru, Ni and Co are calculated to exhibit significant activity (TOF ∼1 s−1). CO2 on the (111) surface is predominantly reduced through the reverse water gas shift (RWGS) reaction, since the production rates of H2O and CO are comparable to the consumption rates of CO2. At the step sites, the hydrogenolysis reaction is more pronounced leading to coke formation. Hydrogenolysis at the step surface also led to significant activity for the reforming reaction. Over the (211) surface, the direct dehydrogenation of ethane to produce ethylene is observed to be predominant. For oxygen assisted ethane dehydrogenation, Co, Ru, Ni and Rh are calculated to show appreciable activity (>1 s−1). The same four metals also show significant CO2 consumption at the step surface. The MKM is further utilised to design bimetallic alloys of Ni and Pt to achieve greater CO2 consumption activity and reduced coke formation with significant activity for the dehydrogenation reaction. While most of the alloys undergo reforming and RWGS reactions, three potential bimetallic combinations (NiFe, NiCo and PtCo) are selected, exhibiting appreciable activity for CO2 assisted dehydrogenation of ethane with some reduction in coke formation, compared to their monometallic counterparts.


 Please wait while we load your content...
Please wait while we load your content...