Recent advances in electrochemical oxidative cross-coupling with hydrogen evolution involving radicals
Abstract
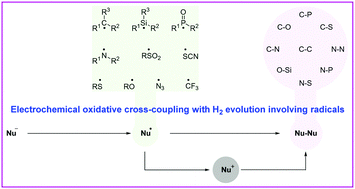
Oxidative cross-coupling has developed into a robust method for carbon–carbon (C–C), carbon–heteroatom (C–X), and heteroatom–heteroatom (X–Y) bond formation. Despite considerable advances in this field, the traditional oxidative cross-coupling reactions usually employ stoichiometric amounts of chemical oxidants to clean up surplus electrons from substrates to form new chemical bonds. Organic electrosynthesis is recognized as an environmentally benign and particularly powerful synthetic platform. Recent advancements have revealed that radical-involved electrochemical oxidative cross-coupling reactions can be achieved under exogenous-oxidant-free conditions. This tutorial review provides an overview of the most recent developments in electrochemical oxidative cross-coupling with hydrogen evolution involving radicals. Emphasis is mainly placed on synthetic and mechanistic aspects. We hope that this tutorial review can promote the development of radical chemistry, electrochemistry, and oxidative cross-coupling reactions.
- This article is part of the themed collection: Radical Chemistry


 Please wait while we load your content...
Please wait while we load your content...