Characterizing hydrogen and tetrel bonds in clusters of CO2 with carboxylic acids†
Abstract
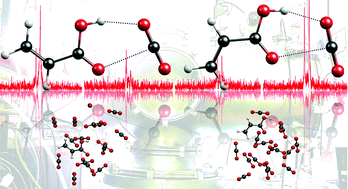
The interaction between carbon dioxide and planar carboxylic acids has been investigated through the analysis of the microwave spectrum of the acrylic acid·CO2 complex and quantum chemical modeling of the R–COOH·(CO2)1,16 clusters, where R = H, CH2CH. As regards the 1 : 1 compounds, two species, involving the s-cis and s-trans conformers of acrylic acid were observed. For both of them, a similar bidentate interaction arises between the carbonyl group of CO2 and the carboxylic group of the organic acid, leading to the formation of a planar six-membered ring. The binding energy is estimated to be De ≃ 21 kJ mol−1, 1/3 being the energy contributions of the tetrel to hydrogen bonds, respectively. In the 1 : 16 clusters, the ring arrangement is broken, allowing for the interaction of the acid with several CO2 molecules. The CO2 molecules completely surround formic acid, whereas, in the case of acrylic acid, they tend to avoid the allyl chain.


 Please wait while we load your content...
Please wait while we load your content...