Accounting for the instantaneous disorder in the enzyme–substrate Michaelis complex to calculate the Gibbs free energy barrier of an enzyme reaction†
Abstract
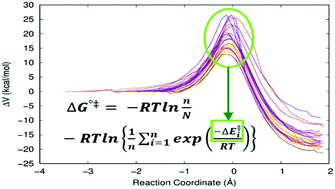
Many enzyme reactions present instantaneous disorder. These dynamic fluctuations in the enzyme–substrate Michaelis complexes generate a wide range of energy barriers that cannot be experimentally observed, but that determine the measured kinetics of the reaction. These individual energy barriers can be calculated using QM/MM methods, but then the problem is how to deal with this dispersion of energy barriers to provide kinetic information. So far, the most usual procedure has implied the so-called exponential average of the energy barriers. In this paper, we discuss the foundations of this method, and we use the free energy perturbation theory to derive an alternative equation to get the Gibbs free energy barrier of the enzyme reaction. In addition, we propose a practical way to implement it. We have chosen four enzyme reactions as examples. In particular, we have studied the hydrolysis of a glycosidic bond catalyzed by the enzyme Thermus thermophilus β-glycosidase, and the mutant Y284P Ttb-gly, and the hydrogen abstraction reactions from C13 and C7 of arachidonic acid catalyzed by the enzyme rabbit 15-lipoxygenase-1.
- This article is part of the themed collection: 2021 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...