Extension of the CAVS model to the simulation of helical peptides in a membrane environment†
Abstract
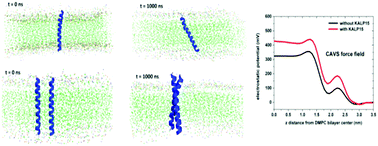
Considering the effect of peptide insertion on the dipole potential of the lipid membrane, we extend the CAVS coarse-grained (CG) model to the simulation of helical peptides in a membrane environment. In this approach, the CG scheme for a peptide backbone is similar to the treatment in the united-atom model, while we treated the side chain of an amino acid by grouping 1–3 heavy atoms into a CG unit. The CAVS CG force field for peptides is optimized by reproducing the experimental results for the backbone (ϕ, ψ) distribution and predicting the PMF profiles of transferring organic molecules in a lipid bilayer membrane obtained from all-atom simulations. The CAVS simulation of a helical peptide in a phosphatidylcholine (PC) lipid bilayer revealed that the insertion of a peptide increases the dipole potential of the PC lipid bilayer, in which the peptide and its neutralized ions make a significant contribution. Finally, we carried out the CAVS simulation for five different helical peptides in the PC lipid bilayer to explore the behavior of peptide tilt, showing excellent agreement with the all-atom simulations. Our work suggests that the peptide tilt should relieve the deformation stress from the lipid bilayer, and the peptide aggregation could reduce the peptide tilt by resisting the deformation stress from the surrounding lipids.


 Please wait while we load your content...
Please wait while we load your content...