The contribution of non-classical CHax⋯OC hydrogen bonds to the anomeric effect in fluoro and oxa-methoxycyclohexanes†
Abstract
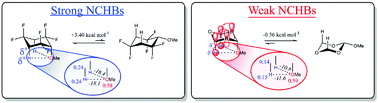
In this theory study we demonstrate the dominance of non-classical 1,3-diaxial CHax⋯OC hydrogen bonds (NCHBs) dictating a ‘pseudo‘ anomeric effect in selectively fluorinated methoxycyclohexanes and also influencing the axial preference in the classical anomeric exhibitor 2-methoxytetrahydropyran, a phenomenon which is most often described as a consequence of hyperconjugation. Analogues of methoxycyclohexane where ring CH2's are replaced by CF2 can switch to an axial preference and theory methods (NBO, QTAIM, NCI) indicate the dominance of 1,3-CHax⋯OMe interactions over hyperconjugation. For 2-methoxytetrahydropyran, it is revealed that the global contribution to the anomeric effect is from electrostatic interactions including NCHBs, not hyperconjugation, although hyperconjugation (nO → σ*CO or nO → σ*CC) remains the main contributor to the exo-anomeric phenomenon. When two and three ether oxygens are introduced into the ring, then both the NCHB interactions and hyperconjugative contributions become weaker, not stronger as might have been anticipated, and the equatorial anomers progressively dominate.
- This article is part of the themed collection: 2021 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...