Thermodynamically stable polymorphs of nitrogen-rich carbon nitrides: a C3N5 study†
Abstract
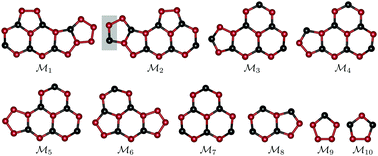
We have carried out an extensive search for stable polymorphs of carbon nitride with C3N5 stoichiometry using the minima hopping method. Contrary to the widely held opinion that stacked, planar, graphite-like structures are energetically the most stable carbon nitride polymorphs for various nitrogen contents, we find that this does not apply for nitrogen-rich materials owing to the high abundance of N–N bonds. In fact, our results disclose novel morphologies with moieties not previously considered for C3N5. We demonstrate that nitrogen-rich compounds crystallize in a large variety of different structures due to particular characteristics of their energy landscapes. The newly found low-energy structures of C3N5 have band gaps within good agreement with the values measured in experimental studies.
- This article is part of the themed collection: 2021 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...