Theoretical study of the pyrolysis of β-1,4-xylan: a detailed investigation on unimolecular concerted reactions†
Abstract
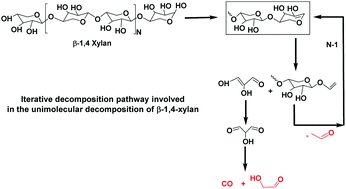
A theoretical study of the thermal decomposition of β-1,4-xylan, a model polymer of hemicelluloses, is proposed for the first time. A mechanism based on unimolecular concerted reactions is elaborated in a comprehensive way. Elementary reactions, such as dehydrations, retro-aldol, retro Diels–Alder, retro-ene, glycosidic bond fissions, isomerizations, etc., are applied to β-1,4-xylan, as well as to the fragments formed. At each stage of the construction of the mechanism, the fragments previously retained are decomposed and the low energy paths are selected to define new fragments. Energy barriers are computed at the CBS-QB3 level of theory and rate coefficients of important reactions are calculated. It is shown that the main reaction pathways can be modelled by reactions involving two specific fragments, which react in closed sequences, similarly to chain-propagating reactions. The proposed reaction scheme allows to predict important species observed during the pyrolysis of xylan, such as aldehydes or CO. In addition, we show that dehydrations require high activation energy and cannot compete with the other reactions. Therefore, it seems difficult to explain, by means of unimolecular homogeneous gas phase reactions, the significant formation of specific species such as furfural as reported by several authors.
- This article is part of the themed collection: 2021 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...