Spontaneous bond dissociation cascades induced by Ben clusters (n = 2,4)†
Abstract
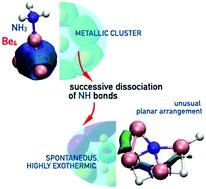
High-level single and multireference ab initio calculations show that the Be4 cluster behaves as a very efficient Lewis acid when interacting with conventional Lewis bases such as ammonia, water or hydrogen fluoride, to the point that the corresponding acid–base interaction triggers a sequential dissociation of all the bonds of the Lewis base. Notably, this behavior is already found for the simplest beryllium cluster, the Be2 dimer. However, whereas for Be2 the first dissociation process involves a low activation barrier which is above the reactants, for Be4 all the bond dissociation processes involve barriers below the entrance channel leading to a cascade of successive exothermic processes, which end up spontaneously in a global minimum in which the bonding patterns of both the base and the Lewis acid are completely destroyed. Indeed, the global minimum, in all cases, is stabilized by three-center Be–H–Be bonds and covalent interactions between the Be atoms and the basic center of the base, which replace the initial metallic bond stabilizing the Be4 cluster. As a consequence, in the global minimum the basic atoms (N, O and F) behave as hyper-coordinated centers. Also importantly, the Be4 cluster and its complexes present RHF–UHF instabilities (not reported before for Be4), which require the use of multireference methods to correctly describe them.
- This article is part of the themed collection: 2021 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...