1′-Ribose cyano substitution allows Remdesivir to effectively inhibit nucleotide addition and proofreading during SARS-CoV-2 viral RNA replication†
Abstract
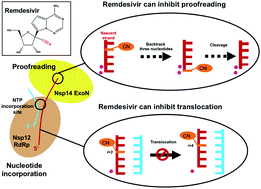
COVID-19 has recently caused a global health crisis and an effective interventional therapy is urgently needed. Remdesivir is one effective inhibitor for SARS-CoV-2 viral RNA replication. It supersedes other NTP analogues because it not only terminates the polymerization activity of RNA-dependent RNA polymerase (RdRp), but also inhibits the proofreading activity of intrinsic exoribonuclease (ExoN). Even though the static structure of Remdesivir binding to RdRp has been solved and biochemical experiments have suggested it to be a “delayed chain terminator”, the underlying molecular mechanisms is not fully understood. Here, we performed all-atom molecular dynamics (MD) simulations with an accumulated simulation time of 24 microseconds to elucidate the inhibitory mechanism of Remdesivir on nucleotide addition and proofreading. We found that when Remdesivir locates at an upstream site in RdRp, the 1′-cyano group experiences electrostatic interactions with a salt bridge (Asp865–Lys593), which subsequently halts translocation. Our findings can supplement the current understanding of the delayed chain termination exerted by Remdesivir and provide an alternative molecular explanation about Remdesivir's inhibitory mechanism. Such inhibition also reduces the likelihood of Remdesivir to be cleaved by ExoN acting on 3′-terminal nucleotides. Furthermore, our study also suggests that Remdesivir's 1′-cyano group can disrupt the cleavage site of ExoN via steric interactions, leading to a further reduction in the cleavage efficiency. Our work provides plausible and novel mechanisms at the molecular level of how Remdesivir inhibits viral RNA replication, and our findings may guide rational design for new treatments of COVID-19 targeting viral replication.
- This article is part of the themed collection: 2021 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...