Structure, dynamics and transport behavior of migrating corrosion inhibitors on the surface of calcium silicate hydrate: a molecular dynamics study
Abstract
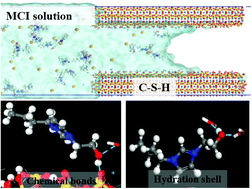
The incorporation of a corrosion inhibitor into a cement-based material can enhance the durability of the reinforced concrete. In this study, molecular dynamics simulation is utilized to study the interfacial structure and dynamic behavior of a solution with three migrating corrosion inhibitors (MCI) functionalized by hydroxyl (–OH), carboxyl (–COO−), and phenyl (–PH) groups in calcium silicate hydrate (CSH) gel pores. The transport rate of inhibitors is greatly dependent on the polarity of the functional group: –PH > –OH > –COO−. The slow migration rate of the inhibitor with –OH and –COO− is attributed to the chemical bond formed between CSH and MCI. The silicate chains near the CSH surface can provide plenty of non-bridging oxygen sites to accept the H-bond from the hydroxyl group in the inhibitor molecule. The surface calcium atom can capture the –COO− by forming an ionic COO–Ca bond. Furthermore, the hydration structure of the inhibitor molecule also influences its transport properties. The inhibitor functionalized by the carboxyl group, associating with the neighboring water molecules, forms ion–water clusters, and the inhibitor molecule and its hydration shell with a long resident time retard the migration rate. Hopefully, this study is able to provide molecules for the development of a migration-type corrosion inhibitor to elongate the service life of cement-based materials.
- This article is part of the themed collection: 2021 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...