H2-oxidation driven by its behavior of losing an electron over B-doped TiO2 under UV irradiation†
Abstract
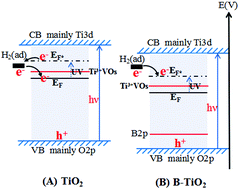
In this work, TiO2 was modified by doping the electron-deficient B element, and then the gas-sensing response of B-TiO2 to H2 under UV irradiation at room temperature in a N2 atmosphere and the oxidation of H2 over B-TiO2 under corresponding conditions were tested. It was found that H2 would accept an electron when adsorbed on the TiO2 surface, while H2 would donate an electron when adsorbed on the B-TiO2 surface. Correspondingly, H2 could not be oxidized over TiO2, but could be oxidized over B-TiO2. This indicated that the oxidation of H2 was dependent on the electron-transfer behavior between H2 and the surface of TiO2 or B-TiO2. Based on the relevant characterization results, it was proposed that H2 could accept an electron from TiO2 due to the higher Fermi level of TiO2, while H2 could donate an electron to B-TiO2 due to the lower Fermi level of B-TiO2 induced by doping B. This indicated that the electron-transfer behavior between H2 and TiO2 could be changed by adjusting the Fermi level of TiO2, while the electron-transfer behavior would further affect the photocatalytic activity of oxidizing H2. This result shows that the doable H2 photocatalytic oxidation in thermodynamics can be controlled by a kinetics factor (H2 losing-an-electron behavior). This work can be applied to provide an understanding of the photocatalytic oxidation behavior of other reactants over semiconductor materials.
- This article is part of the themed collection: 2021 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...