Probing the electronic and ionic transport in topologically distinct redox-active metal–organic frameworks in aqueous electrolytes†
Abstract
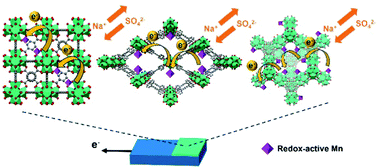
Three topologically distinct zirconium-based metal–organic frameworks (Zr-MOFs) constructed from redox-innocent linkers, MOF-808, defective UiO-66, and CAU-24, are synthesized, and the spatially dispersed redox-active manganese sites are post-synthetically immobilized on the hexa-zirconium nodes of these Zr-MOFs. The crystallinity, morphology, porosity, manganese loading, and bulk electrical conductivity of each material are studied. The redox-hopping-based electrochemical reaction between the installed Mn(III) and Mn(IV) occurring within the thin films of these MOFs in aqueous electrolytes is investigated, in the presence of various concentrations of Na2SO4 in the electrolytes. Cyclic voltammetry is used to qualitatively study the redox-hopping process, and chronoamperometry is used to quantify the electrochemically active fractions of manganese sites within the MOF thin film as well as the values of apparent diffusivity for the redox-hopping process. By adjusting the concentration of Na2SO4 in the electrolyte, the rate-determining step for the redox-hopping process can be tuned from ionic transport to electronic transport, and the Mn-decorated MOF-808, which possesses the largest pore size, can achieve the highest value of apparent diffusivity. Findings here shed light on the selection of Zr-MOF as well as the choice of electrolyte concentration for the applications of MOFs in supercapacitors and electrocatalysis relying on such redox-hopping processes in aqueous electrolytes.


 Please wait while we load your content...
Please wait while we load your content...