Interfacial acidity on the strontium titanate surface: a scaling paradigm and the role of the hydrogen bond†‡
Abstract
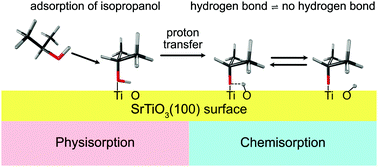
A fundamental understanding of acidity at an interface, as mediated by structure and molecule–surface interactions, is essential to elucidate the mechanisms of a range of chemical transformations. While the strength of an acid in homogeneous gas and solution phases is conceptually well understood, acid–base chemistry at heterogeneous interfaces is notoriously more complicated. Using density functional theory and nonlinear vibrational spectroscopy, we present a method to determine the interfacial Brønsted–Lowry acidity of aliphatic alcohols adsorbed on the (100) surface of the model perovskite, strontium titanate. While shorter and less branched alkanols are known to be less acidic in the gas phase and more acidic in solution, here we show that shorter alcohols are less acidic whereas less substituted alkanols are more acidic at the gas–oxide interface. Hydrogen bonding plays a critical role in defining acidity, whereas structure–acidity relationships are dominated by van der Waals interactions between the alcohol and the surface.
- This article is part of the themed collection: 2021 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...