Solvent effect on the competition between weak and strong interactions in phenol solutions studied by near-infrared spectroscopy and DFT calculations†
Abstract
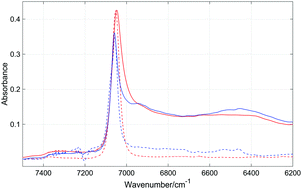
Near-infrared (NIR) spectra of phenol in a series of non-aromatic and aromatic solvents were recorded to study the competition between various types of solute–solute and solute–solvent interactions. Depending on the phenol concentration, the free OH and OH involved in the OH⋯OH interactions in the dimers and higher associates are present in cyclohexane solutions. On the other hand, free OH does not appear in Cl-containing solvents since at a low phenol content the OH groups participate in the OH⋯Cl interactions. In CCl4 and tetrachloroethylene this interaction is weak, while in chlorobenzene the strength of this interaction is higher. In the aromatic solvents the solute–solute OH⋯OH interactions compete with the solute–solvent OH⋯π and aromatic CH⋯OH ones. Consequently, the degree of self-association of phenol in aromatic solvents is smaller than that in non-aromatic ones. The strength of the OH⋯π interactions increases with growing electron-donating ability of the substituents in the benzene derivatives. This observation obtained from the NIR spectra is in line with the results of the theoretical calculations (DFT). A clear correlation appears between the number of methyl groups in aromatic solvents and the population of the free OH groups. The methyl groups are steric hindrances and impede the formation of the OH⋯OH and OH⋯π interactions. Our results suggest the presence of aromatic CH⋯OH solute–solvent interactions, not observed in previous studies. NIR spectroscopy appears to be a powerful tool for exploration of free and weakly-bonded OH groups.


 Please wait while we load your content...
Please wait while we load your content...