Potassium and sodium ion complexes with a partial peptide of the selectivity filter in K+ channels studied by cold ion trap infrared spectroscopy: the effect of hydration†
Abstract
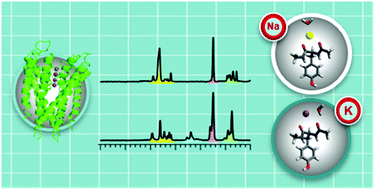
Potassium channels allow K+ to rapidly diffuse, while the selectivity filter (SF) actively blocks Na+. The presence of water in the SF during ion translocation remains under debate due to the experimental and computational challenges in characterizing the interactions between water, ions, and the SF. Our bottom-up approach has been applied to a system composed of a partial peptide of the SF (Ac-tyrosine-NHMe) with a metal ion and a single water molecule to probe these interactions. The IR photodissociation spectra of M+Ac-tyrosine-NHMe(H2O) (M = Na, K) combined with quantum chemical calculations revealed that the water molecule binding sites are ion-dependent. In addition, the ion–peptide distances are elongated significantly for the K+ complex in comparison to the Na+ complex by the addition of a single water molecule. This striking structural difference with the water molecule is discussed in relation to ion selectivity and translocation within the K+ channel.


 Please wait while we load your content...
Please wait while we load your content...