Pair of chiral molecular ladders and successive hydration in single-crystal-to-single-crystal mode†
Abstract
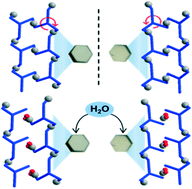
Unusual successive two-step crystallization of a pair of chiral coordination polymers was carried out. Self-assembly of Zn(NO3)2 with a pair of chiral C3-symmetric tridentate N-donor ligands (1S,2R-L and 1R,2S-L) produces single crystals consisting of chiral enantiomeric 1D ladder coordination polymers, CH2Cl2·2CH3CN@[Zn3(NO3)6(1S,2R-L)2] and CH2Cl2·2CH3CN@[Zn3(NO3)6(1R,2S-L)2], after which the single crystals are transformed into new crystals in their hydrated forms, 2CH3CN@[Zn3(NO3)6(1S,2R-L)2(H2O)2] and 2CH3CN@[Zn3(NO3)6(1R,2S-L)2(H2O)2], respectively, under the moisture condition, in the single-crystal-to-single-crystal (SCSC) mode. The crystals consist of hexagonally packed chiral 1D coordination polymers in an enantiomeric arrangement, and their hydration shows a subtle difference depending on the chirality.
- This article is part of the themed collection: Crystal Growth


 Please wait while we load your content...
Please wait while we load your content...