Fluorine as a robust balancer for tuning the reactivity of topo-photoreactions of chalcones and the photomechanical effects of molecular crystals†
Abstract
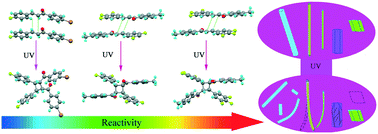
Fluorine-free chalcones and chalcones bearing different numbers of fluorine atoms have been synthesized. It is found that fluorine can tune the reactivity of photo-induced [2 + 2] cycloaddition reactions in crystals. The higher the number of fluorine atoms, the higher the reactivity of the photodimerization. The single crystal structures and Hirshfeld surface analysis illustrated that the introduction of fluorine not only increased the molecular planarity but could also steer the potentially reactive double bonds in appropriate positions of the crystal lattices to meet Schmidt's criteria. Therefore, the stereospecific [2 + 2] cycloaddition reactions took place to afford one diastereoisomer from the reactive chalcones except for (E)-3-(4-fluorophenyl)-1-phenylprop-2-en-1-one (1FChH). Additionally, the chalcone-based molecular crystals exhibited various photomechanical behaviors, such as bending toward or away from the light source, swinging, cracking and jumping, driven by topo-photoreactions. As expected, the more efficient photo-induced [2 + 2] cycloaddition reactions in crystalline states led to more significant motions of the molecular crystals. Powder X-ray diffraction results suggested that the solid state [2 + 2] cycloaddition reactions changed the unit cell of the single crystals. The photo-induced bending toward or away from the light source for the needle-like crystals originated from the contraction or the expansion of the phototropic surface. Hence, the robust balancer fluorine in chalcones might play an important role in the crystalline packing. This provides a facile approach in crystal engineering to fabricate photo-induced mechanically responsive crystalline materials.
- This article is part of the themed collections: Crystal Engineering Techniques and Mechanically responsive crystalline materials


 Please wait while we load your content...
Please wait while we load your content...