Growth kinetic control over MgFe2O4 to tune Fe occupancy and metal–support interactions for optimum catalytic performance†
Abstract
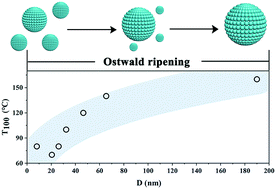
Manipulating the growth behavior of nano-sized supports is proved to have extremely favorable effects for optimizing the performance of hybrid catalysts. Herein, we investigated the growth kinetics of MgFe2O4 and revealed its size-dependent behavior of Fe occupancies at octahedral and tetrahedral sites and CO oxidation catalytic activity for the first time. MgFe2O4, with particle size ranging from 8.1 nm to 190.0 nm, was prepared via a sol–gel method after post-annealing treatment at a temperature from 400 °C to 1000 °C. The growth of MgFe2O4 nanoparticles obeys the Ostwald ripening mechanism with the quantitative formula D5 = 2.08 × 1014t exp(−122.13/RT). The systematic characteristics show that the redox behavior and O2 activation of hybrid catalysts of Pt/MgFe2O4-T rely on the grain size of MgFe2O4 nanoparticles. The Pt/MgFe2O4-500 catalyst with a support size of 20.6 nm exhibits a remarkable CO oxidation performance due to its abundant chemisorbed oxygen species on the surface and enhanced metal–support interactions among all examined catalysts. Our findings offer significant insight into the size-dependent structure, redox ability and oxygen activation properties of spinel materials and can greatly impact future exploration of novel materials in heterogeneous catalysis.
- This article is part of the themed collection: Crystal Growth


 Please wait while we load your content...
Please wait while we load your content...