Alkyl-substituted bis(4-((9H-fluoren-9-ylidene)methyl)phenyl)thiophenes: weakening of intermolecular interactions and additive-assisted crystallization†
Abstract
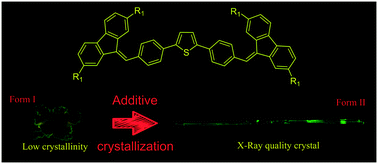
Aggregation-induced emission (AIE) materials find their applications in organic optoelectronics, bio-imaging and sensors. In this work, we introduced alkyl substituents in AIE-active bis(4-((9H-fluoren-9-ylidene)methyl)phenyl)thiophene and elucidated their effect on the molecular and crystal structures, crystallization and optical properties of materials. Ethyl-containing (C2-BFMPT) and octyl-containing (C8-BFMPT) 2,5-bis(4-((2,7-dialkyl-9H-fluoren-9-ylidene)methyl)phenyl)thiophenes were synthesized in 4 steps. The introduction of alkyl groups weakened intermolecular interactions, decreased the crystal quality, melting point, and density, and increased the solubility of materials. Octyl-containing derivative was demonstrated to generate two crystal forms obtained by the native (form I) and additive-assisted (form II) crystallizations. The latter appeared to have a better crystal quality. C–H⋯π interactions and an extensive positional disorder were revealed for both derivatives. In C8-BFMPT crystals (form II) only a half of molecular backbones were well localized due to multiple intermolecular C–H⋯π interactions, whereas another half demonstrated a high positional disorder. The AIE effect with a negligible photoluminescence (PL) quantum yield (QY) in solution and a PL QY of 5% for C2-BFMPT and 2% for C8-BFMPT (form II) crystals was demonstrated. The cooling of the C8-BFMPT form II resulted in 10-fold increase of PL QY. The introduction of alkyl-substituents and additive-assisted crystallization are highlighted as powerful tools for the control of crystal packing, morphology, polymorphism and the optical performance of AIE-materials.
- This article is part of the themed collection: Crystal Engineering Techniques


 Please wait while we load your content...
Please wait while we load your content...