Growth of LaCoO3 crystals in molten salt: effects of synthesis conditions†
Abstract
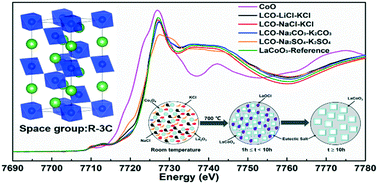
Molten salts have been reported to be excellent media for the preparation of multicationic oxides, which require harsh conditions to crystallize. Molten salt synthesis provides a wider operational temperature range (usually extends to 1000 °C) and faster mass transport and nucleation processes, thus allowing the preparation of crystalline solids, which require higher temperatures, such as perovskites. However, the mechanisms that control the solid-state formation and phase selection are poorly understood. Herein, several typical molten alkali metal halide (Cl−) and oxosalt (CO32− and SO42−) systems were employed as the reaction media for the synthesis of LaCoO3 (LCO) crystals, and the effects of ionic salt species on the morphological, geometric and electronic structures of LCO crystals were examined by using X-ray diffraction (XRD), scanning electron microscopy (SEM) and X-ray absorption spectroscopy (XAS). The LCO perovskite was successfully synthesized in a molten NaCl–KCl eutectic mixture at 700 °C, which was lower than that used for the conventional mixed oxide synthesis. Our results show that in the molten salt synthesis, the type of reaction medium, calcination temperature, the ratio of salt, and reaction time govern the microscopic mechanisms in the preparation of multicationic oxides. Most importantly, we observed that LaOCl was formed as an intermediate, which plays a key role in the reaction pathway for the phase-selective synthesis of perovskite LCO.
- This article is part of the themed collection: Crystal Growth


 Please wait while we load your content...
Please wait while we load your content...