High dual-state blue emission of a functionalized pyrazoline derivative for picric acid detection†
Abstract
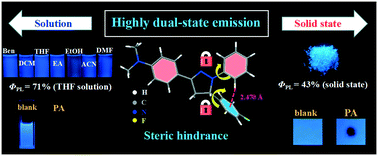
Developing dual-state fluorescence materials with high emission efficiency both in solution and solid states is still a challenge. Herein, a functionalized pyrazoline derivative (NF) was designed and synthesized. NF exhibited high blue emission efficiency in both THF solution and solid states with the absolute fluorescence quantum yields of 58% and 43%, respectively. The mechanism of this unique fluorescence behavior was systematically studied with the help of single-crystal structure analysis. The results showed that a close-knit molecular structure existed due to intramolecular C–H⋯π interactions, which can impair the intramolecular rotation to accelerate the radiative transition in the mono-molecule state (in dilute solution). Moreover, the steric hindrance and intermolecular hydrogen-bond interactions of the substituent groups can restrict the close packing and prompt the formation of J-aggregates in the aggregation state, which resulted in high emission performance in the solid state. Meanwhile, the dual-state blue emission property of NF was utilized for the picric acid (PA) detection in the solution state and on a test paper by the synergistic effects of photo-induced electron transfer (PET) and Förster resonance energy transfer (FRET) processes. The limit of detection as low as 6.80 × 10−7 M achieved in the THF–H2O mixture. This work provided a new scope for the design of high dual-state blue emission materials.
- This article is part of the themed collection: Database Analysis


 Please wait while we load your content...
Please wait while we load your content...