Harnessing the electronic differences between CAAC-stabilised 1,4-diborabenzene and 9,10-diboraanthracene for synthesis†
Abstract
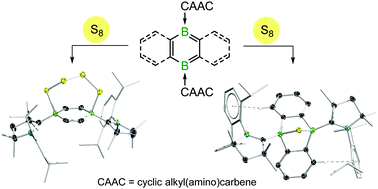
The oxidation of doubly cyclic alkyl(amino)carbene-stabilised closed-shell 1,4-diborabenzene with sulfur or selenium yields S4/S5- or Se4-bridged hexa-1,4-dienes, respectively, whereas that of the related open-shell singlet biradical 9,10-diboraanthracene with O2, sulfur or selenium yields the endoperoxo- or S/Se-bridged bicyclic species, respectively.
- This article is part of the themed collection: Boron Chemistry in the 21st Century: From Synthetic Curiosities to Functional Molecules


 Please wait while we load your content...
Please wait while we load your content...