PN-Doped tetraphenylnaphthalene: a straightforward synthetic strategy analogous to BN-annulation†
Abstract
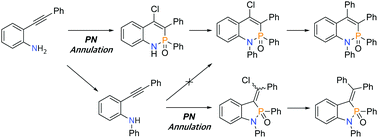
Compared to BN heterocycles, few studies on PN heterocycles have been reported to date. Herein, we developed an efficient synthetic strategy analogous to BN-annulation to simultaneously incorporate a PN bond and a halogen group into the naphthalene core. Subsequently, we prepared PN-containing tetraphenylnaphthalene using this method, followed by palladium-catalyzed cross-coupling and reduction reactions. The prepared molecule was characterized via X-ray crystallography, NMR spectroscopy, UV-vis spectroscopy, and cyclic voltammetry.
- This article is part of the themed collection: 2021 Emerging Investigators


 Please wait while we load your content...
Please wait while we load your content...