C2-Selective silylation of pyridines by a rhodium–aluminum complex†
Abstract
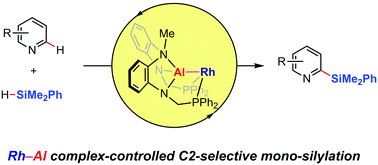
We have developed a C2-selective mono-silylation of a variety of pyridines using a Rh–Al complex. Both the site- and mono-selectivity are controlled via the pyridine coordination to the Lewis-acidic Al center prior to the activation of the pyridine C(2)–H bond at the proximal Rh center. A reaction mechanism is proposed based on several mechanistic studies, including the isolation of a (2-pyridyl)silylrhodium intermediate.
- This article is part of the themed collection: Chemical Communications HOT Articles


 Please wait while we load your content...
Please wait while we load your content...