Modulating alkene reactivity from oxygenation to halogenation via electrochemical O2 activation by Mn porphyrin†
Abstract
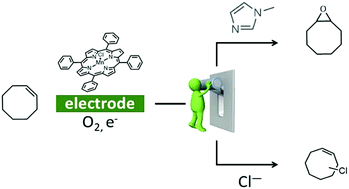
Oxidation of organic substrates is achieved in nature under mild conditions thanks to metalloenzymes but remains a challenge for chemists. Herein we show by UV-Vis spectroelectrochemistry that when MnIIITPPCl is electrochemically reduced to MnII in CH2Cl2 under O2, a MnIIO2˙ species is generated. Benzoic anhydride reacts with the latter triggering a catalytic current in cyclic voltammetry. Electrolysis on the catalytic wave in the presence of cyclooctene leads to its oxygenation or halogenation depending on the axial ligand present as reported here for the first time.
- This article is part of the themed collections: Chemical Communications HOT articles and Bioinspired metal complexes for chemical transformations and catalysis


 Please wait while we load your content...
Please wait while we load your content...