Analysis of protein phosphorylation in solution and in cells by using an ATP analogue in combination with fluorescence techniques†
Abstract
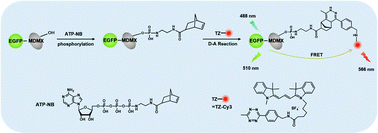
Protein phosphorylation is a very important mechanism for regulating and controlling the activity and function of proteins, and is closely associated with signal transduction, gene expression, cell cycle and other life activities in organisms. In this paper, we proposed a new strategy for studying protein phosphorylation in living cells by combining fluorescence resonance energy transfer (FRET) with a small molecule adenosine 5′-triphosphate (ATP) analogue. We synthesized a new ATP analogue functionalized by norbornene (ATP-NB), and a tetrazine modified fluorescent probe Cyanine3 (TZ-Cy3). Based on the inverse electron demand Diels–Alder (D–A) reaction, ATP-NB phosphorylated proteins in solution and in living cells were in situ labelled with TZ-Cy3. By combining FRET with fluorescence correlation spectroscopy (FRET-FCS) and imaging technology, we established an efficient method for studying the phosphorylation of proteins in solution and in living cells using an ATP analogue instead of natural ATP. We studied the effects of phosphatase inhibitors on the phosphorylation of proteins in living cells. Our results documented that ATP-NB is a small molecule ATP analogue with hydrophobicity, which can penetrate cells and efficiently phosphorylate proteins in living cells. This strategy is well suitable for in situ study of protein phosphorylation in living cells.
- This article is part of the themed collection: Analyst HOT Articles 2021


 Please wait while we load your content...
Please wait while we load your content...