Total synthesis of diplofuranone A and diapolic acid A†
Abstract
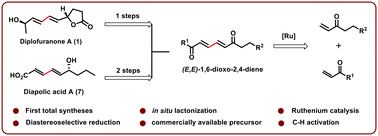
The first and concise syntheses of the anticancer agent diplofuranone A and the fatty acid-derived metabolite diapolic acid A have been demonstrated using easily accessible and commercially available starting materials. The key feature of these syntheses is the efficient diversification of highly stereo- and chemoselectively constructed (E,E)-1,6-dioxo-2,4-dienes using ruthenium catalytic conditions, which enabled straightforward access to diversely substituted bioactive molecules.


 Please wait while we load your content...
Please wait while we load your content...