Effect of water frustration on water oxidation catalysis in the nanoconfined interlayers of layered manganese oxides birnessite and buserite†
Abstract
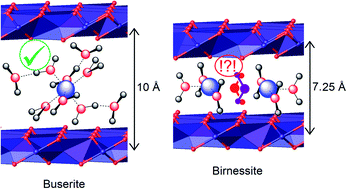
The role of geometric frustration of water molecules in the rate of water oxidation in the nanoconfined interlayer of manganese-oxide layered materials (birnessite, buserite) is examined in a well-controlled experiment. Calcium buserite is prepared, and used in a split-batch synthetic protocol to prepare calcium birnessite, sodium buserite, and sodium birnessite, and partially dehydrated sodium birnessite. Thus, four samples are prepared in which features effecting catalytic efficiency (defect density, average manganese oxidation state) are controlled, and the main difference is the degree of hydration of the interlayer (two layers of water in buserites vs. one layer of water in birnessite). Molecular dynamics simulations predict birnessite samples to exhibit geometric water frustration, which facilitates redox catalysis by lowering the Marcus reorganization energy of electron transfer, while buserite samples exhibit traditional intermolecular hydrogen bonding among the two-layer aqeuous region, leading to slower catalytic behavior akin to redox reactions in bulk water. Water oxdiation activity is investigated using chemical and electrochemical techniques, demonstrating and quantifying the role of water frustration in enhancing catalysis. Calculation and experiment demonstrate dehydrated sodium birnessite to be most effective, and calcium buserite the least effective, with a difference in electrocatlytic overpotential of ∼750 mV and a ∼20-fold difference in turnover number.


 Please wait while we load your content...
Please wait while we load your content...
