Enhanced electrocatalytic activity of in situ carbon encapsulated molybdenum phosphide derived from a hybrid POM for the HER over a wide pH range†
Abstract
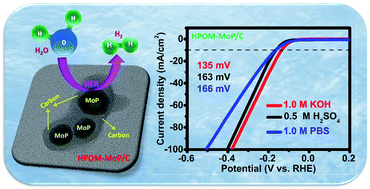
Increasing environmental pollution and the rapid exhaustion of fossil fuels have led to the exploration of alternative energy sources. Recently, hydrogen being a clean, and carbon-free energy carrier has been considered as the next generation transportation fuel. Electrochemical reduction of water is a simple, carbon-dioxide free technique for hydrogen production. Herein, we report a hybrid polyoxometalate derived in situ carbon encapsulated molybdenum phosphide (HPOM-MoP/C) electrocatalyst for the hydrogen evolution reaction (HER). The X-ray diffraction (XRD) pattern confirms the formation of highly crystalline pure-phase molybdenum phosphide (MoP) nanoparticles. The morphology and size of the synthesized material were characterized by field emission scanning electron microscopy (FESEM), transmission electron microscopy (TEM) and high-resolution transmission electron microscopy (HRTEM) techniques. Notably, the obtained MoP nanoparticles exhibited excellent HER activity and stability in a wide range of pH. This HPOM-MoP/C electrode shows low overpotentials of 135, 163 and 166 mV at a current density of 10 mA cm−2 in alkaline (pH ∼14), acidic (pH ∼0) and neutral (pH ∼7), electrolytes respectively.
- This article is part of the themed collection: Sustainable Energy & Fuels Recent HOT Articles


 Please wait while we load your content...
Please wait while we load your content...