Azidosphinganine enables metabolic labeling and detection of sphingolipid de novo synthesis†
Abstract
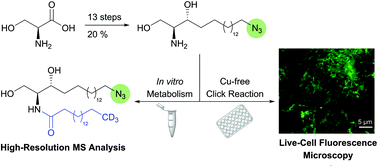
Here were report the combination of biocompatible click chemistry of ω-azidosphinganine with fluorescence microscopy and mass spectrometry as a powerful tool to elaborate the sphingolipid metabolism. The azide probe was efficiently synthesized over 13 steps starting from L-serine in an overall yield of 20% and was used for live-cell fluorescence imaging of the endoplasmic reticulum in living cells by bioorthogonal click reaction with a DBCO-labeled fluorophore revealing that the incorporated analogue is mainly localized in the endoplasmic membrane like the endogenous species. A LC-MS(/MS)-based microsomal in vitro assay confirmed that ω-azidosphinganine mimics the natural species enabling the identification and analysis of metabolic breakdown products of sphinganine as a key starting intermediate in the complex sphingolipid biosynthetic pathways. Furthermore, the sphinganine-fluorophore conjugate after click reaction was enzymatically tolerated to form its dihydroceramide and ceramide metabolites. Thus, ω-azidosphinganine represents a useful biofunctional tool for metabolic investigations both by in vivo fluorescence imaging of the sphingolipid subcellular localization in the ER and by in vitro high-resolution mass spectrometry analysis. This should reveal novel insights of the molecular mechanisms sphingolipids and their processing enzymes have e.g. in infection.
- This article is part of the themed collection: Chemical Biology in OBC


 Please wait while we load your content...
Please wait while we load your content...