Structure–activity relationship of surface hydroxyl groups during NO2 adsorption and transformation on TiO2 nanoparticles†
Abstract
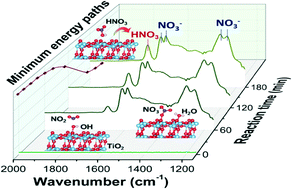
The role of hydroxyl groups (OH) is significant in understanding the surface reactions on oxides. Here we combined spectroscopic experiments and theoretical calculations to reveal the role of OH in the heterogeneous reactions of NO2 on TiO2 nanoparticles with various crystal structures. The interaction between OH and NO2 was determined to be a critical step, giving rise to the formation of surface HNO3. HNO3 was found to be either a stable product on amorphous TiO2, or an important intermediate of nitrate on anatase due to its further reaction with nearby OH. The lack of surface OH on rutile limited its reactivity toward NO2. This study presents clear evidence that the reactivity of TiO2 toward NO2 greatly depends on the surface OH as well as the crystalline form. Considering the ubiquitous presence of TiO2 nanoparticles in catalysts and in natural environments, our results could provide insights helpful to future research in both environmental catalysis and atmospheric chemistry.


 Please wait while we load your content...
Please wait while we load your content...