Effect of Ru substitution on the first charge–discharge cycle of lithium-rich layered oxides†
Abstract
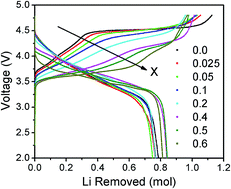
The electrochemical properties of lithium-rich layered oxides are affected significantly by the first-cycle charge–discharge characteristics. Specifically, the lengths of the sloping and plateau regions of the first charge influence the charge capacity, irreversible capacity loss in the first cycle, reversible capacity, cyclability, and voltage decay. We present here an analysis and understanding of the first charge–discharge profiles by substituting Ru4+ for Mn4+ in Li1.2Mn0.6−xRuxNi0.2O2 (0 ≤ x ≤ 0.60). While most of the X-ray diffraction peaks at low Ru contents could be indexed on the basis of the trigonal R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m structure, those for x ≥ 0.4 could be indexed on the basis of the monoclinic P12/m structure of Li2RuO3. Rietveld refinement data provides preliminary evidence of Ru–Ru dimer formation in these samples. The Ru substitution increases the sloping region, but decreases the plateau region corresponding to the loss of oxygen from the lattice. The shorter plateau region decreases the charge capacity and irreversible capacity loss. The dQ/dV plots and open-circuit voltage measurements indicate a decrease in cell voltage with increasing Ru substitution, largely due to the shifts in the positions of the Ru4+/5+:4d and O2−:2p bands. Ru–Ru dimerization that splits the Ru4+:t2g band into bonding/antibonding orbitals could explain the decrease in voltage and shortening of the oxygen-loss plateau due to a decreased overlap between the Ru4+/5+:4d and O2−:2p bands.
m structure, those for x ≥ 0.4 could be indexed on the basis of the monoclinic P12/m structure of Li2RuO3. Rietveld refinement data provides preliminary evidence of Ru–Ru dimer formation in these samples. The Ru substitution increases the sloping region, but decreases the plateau region corresponding to the loss of oxygen from the lattice. The shorter plateau region decreases the charge capacity and irreversible capacity loss. The dQ/dV plots and open-circuit voltage measurements indicate a decrease in cell voltage with increasing Ru substitution, largely due to the shifts in the positions of the Ru4+/5+:4d and O2−:2p bands. Ru–Ru dimerization that splits the Ru4+:t2g band into bonding/antibonding orbitals could explain the decrease in voltage and shortening of the oxygen-loss plateau due to a decreased overlap between the Ru4+/5+:4d and O2−:2p bands.

 Please wait while we load your content...
Please wait while we load your content...