Sequential acid-catalyzed alkyl glycosylation and oligomerization of unprotected carbohydrates†
Abstract
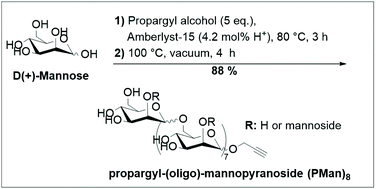
An efficient method has been developed to synthesize end-functionalized oligosaccharides from unprotected monosaccharides in a one-pot/two-step approach. In the first step, mannose (and glucose) was functionalized with an alkyne group at the anomeric position through the Fisher-glycosylation reaction with propargyl alcohol as a glycosyl acceptor. In the second step, the functionalized monosaccharides were oligomerized and the experimental conditions were optimized by varying the temperature, time and the molar ratio between alcohol and sugar to reach a 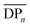 up to 8. The obtained oligosaccharides showed complete propargylation at their reducing chain-end and were successfully coupled to oleic acid via the Huisgen reaction, affording bio-based surfactants.
up to 8. The obtained oligosaccharides showed complete propargylation at their reducing chain-end and were successfully coupled to oleic acid via the Huisgen reaction, affording bio-based surfactants.


 Please wait while we load your content...
Please wait while we load your content...