Electrocatalytic syngas generation with a redox non-innocent cobalt 2-phosphinobenzenethiolate complex†
Abstract
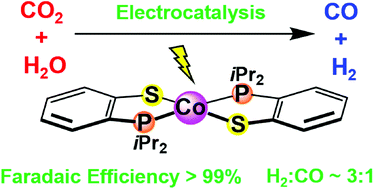
A cobalt complex supported by the 2-(diisopropylphosphaneyl)benzenethiol ligand was synthesized and its electronic structure and reactivity were explored. X-ray diffraction studies indicate a square planar geometry around the cobalt center with a trans arrangement of the phosphine ligands. Density functional theory calculations and electronic spectroscopy measurements suggest a mixed metal–ligand orbital character, in analogy to previously studied dithiolene and diselenolene systems. Electrochemical studies in the presence of 1 atm of CO2 and Brønsted acid additives indicate that the cobalt complex generates syngas, a mixture of H2 and CO, with faradaic efficiencies up to >99%. The ratios of H2 : CO generated vary based on the additive. A H2 : CO ratio of ∼3 : 1 is generated when H2O is used as the Brønsted acid additive. Chemical reduction of the complex indicates a distortion towards a tetrahedral geometry, which is rationalized with DFT predictions as attributable to the populations of orbitals with σ*(Co–S) character. A mechanistic scheme is proposed whereby competitive binding between a proton and CO2 dictates selectivity. This study provides insight into the development of a catalytic system incorporating non-innocent ligands with pendant base moieties for electrochemical syngas production.


 Please wait while we load your content...
Please wait while we load your content...